
Review of common medicines used to treat hypertension – valsartan – due to detection of impurity, N-nitrosodimethylamine (NDMA), a probable human carcinogen! (Part VIII)
- 29 мая 2019
- 4-(methyl)(nitroso)amino)butanoic acid, active substance, angiotensin-II-receptor antagonists, angiotensin-II-receptor blockers, API, ARBs, Article 31, azilsartan, cancer, candesartan, carcinogen, CEP, conditions to MA, diabetes, DIPNA, drug product, EC, EDQM, EIPNA, EMA, eprosartan, European Commission, Hetero Labs, human, hypertension, impurity, interim, investigation, irbesartan, legally binding decision, limit, losartan, MA, MAH, manufacturer, manufacturing, manufacturing process, manufacturing site, marketing authorisation holder, medicinal product, medicines, N-nitrosodiethylamine, N-nitrosodiisopropylamine, N-nitrosodimethylamine, N-nitrosoethylisopropylamine, NDEA, NDMA, nitrosamine, NMBA, olmesartan, pioglitazone, review, root cause, sartans, scientific conclusion, specific ring structure, suspension, telmisartan, temporary, tetrazole, valsartan, Zhejiang Huahai Pharmaceuticals,

IMPORTANT! Update [28/10/2020]
Having considered the knowledge acquired on the presence of nitrosamines in medicinal products since the sartans referral and taking into account the data assessed within the review under Article 5(3), in particular related to the root causes where it became clear that the root causes can be numerous, concomitant, at any stage of the production or storage of the medicinal product and cannot always be characterised, the CHMP considered that the outcome of the sartan referral should be reconsidered in light of the outcome of Art. 5(3) referral.
CHMP opinion on the impact of the Article 5(3) referral on nitrosamines in human medicinal products on the referral under article 31 of Directive 2001/83/EC for sartans medicinal products containing a tetrazole ring is expected in November 2020.
Update [14/12/2020]
EMA’s human medicines committee (CHMP) has aligned recommendations for limiting nitrosamine impurities in sartan medicines with recent recommendations it issued for other classes of medicines.
The main change concerns the limits for nitrosamines, which previously applied to the active ingredients but will now apply instead to the finished products (e.g. tablets). These limits, based on internationally agreed standards (ICH M7(R1)), should ensure that the excess risk of cancer from nitrosamines in any sartan medicines is below 1 in 100,000 for a person taking the medicine for lifelong treatment.
In line with previous recommendations, companies should have appropriate control strategies to prevent or limit the presence of nitrosamine impurities as much as possible and, where necessary, improve their manufacturing processes. Companies should also evaluate the risk of nitrosamines being present in their medicines and carry out appropriate tests.
NEW! Update [03/03/2021]
COMMISSION IMPLEMENTING DECISION of 19.2.2021 amending Commission Decision C(2019) 2698 of 2 April 2019 concerning, in the framework of Article 31 of Directive 2001/83/EC of the European Parliament and of the Council, the marketing authorisations of medicinal products for human use which contain the active substances “candesartan”, “irbesartan”, “losartan”, “olmesartan”, “valsartan” is available here and Annexes (Annex I Scientific conclusions and Annex II Amendments to the conditions to the marketing authorisation(s)) are available here.
On 31 January 2019, EMA recommended that companies making sartan blood pressure medicines (also known as angiotensin II receptor blockers, ARBs) review their manufacturing processes so that they do not produce nitrosamine impurities.
An initial investigation report on the root cause of the presence of N-nitrosodimethylamine (NDMA) provided by Zhejiang Huahai Pharmaceutical, China upon request from the supervisory authority in Italy (AIFA), indicated that NDMA is formed at the tetrazole ring-forming step in ZH’s valsartan API manufacturing process, and the level of NDMA present may depend on the reaction conditions used.
It should be reminded here, that the review of valsartan medicines was triggered by the European Commission on 5 July 2018 under Article 31 of Directive 2001/83/EC. On 20 September 2018, the review was extended to include medicines containing candesartan, irbesartan, losartan and olmesartan.
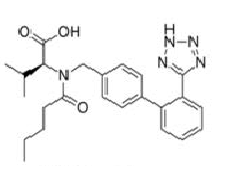
Angiotensin-II-receptor antagonists/blockers subject to the procedure (tetrazole group circled in red at the example of losartan):
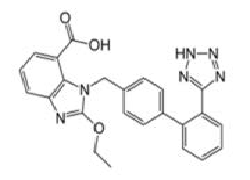
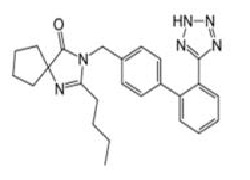
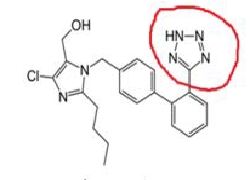
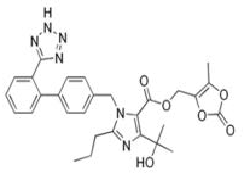
Scientific conclusions related to the procedure are presented in Annex I while Conditions to the marketing authorisation(s) in Annex II.
It should be noted however, that there are additional ARBs not containing a tetrazole group authorised in the EU, and hence these are not subject to this procedure:
- Azilsartan,
- Eprosartan,
- Telmisartan.
Temporary limits for NDMA, NDEA as well as for NMBA, DIPNA and EIPNA impurities have been set in line with current ICH M7(R1) guidelines and are given in the table below.
NDMA, NMBA
NDEA, DIPNA, EIPNA
Active substance
Maximum daily
dose [mg]
Acceptable Intake [ng/day]
Limit [ppm]
Acceptable Intake [ng/day]
Limit [ppm]
Candesartan
32
96.0
3.000
26.5
0.820
Irbesartan
300
96.0
0.320
26.5
0.088
Losartan
150
96.0
0.640
26.5
0.177
Olmesartan
40
96.0
2.400
26.5
0.663
Valsartan
320
96.0
0.300
26.5
0.082
NDMA – N-nitrosodimethylamine
NDEA – N-nitrosodiethylamine,
NMBA – 4-(methyl)(nitroso)amino)butanoic acid
DIPNA – N-nitrosodiisopropylamine
EIPNA – N-nitrosoethylisopropylamine
EMA’s recommendations for NDMA and NDEA were sent to the European Commission, which issued a legally binding decisions.
We have already informed on our website about the ongoing review of valsartan and other ‘sartan’ medicines due to the detection of impurity N-nitrosodimethylamine (NDMA) as well as suspensions of CEPs by EDQM in Part I, Part II, Part III, Part IV, Part V, Part VI and Part VII entries.

In addition, as part of strengthened monitoring of manufacturing, EMA and national authorities are also requesting as precaution that companies using certain reagents to manufacture the diabetes medicine pioglitazone test their products and check their processes to rule out the presence of nitrosamine impurities, in particular nitrosodimethylamine (NDMA).
The request follows the detection of low levels of NDMA in a few batches of pioglitazone manufactured by Hetero Labs in India, which were within strict limits previously set for sartans (refer to Annex I above) and are considered acceptably safe.
To check the current status of CEP for pioglitazone hydrochloride please click here.
Sources: EMA, EDQM
Updated: 2020-10-28
Related entries:





